 FEATURED COMPANY
FEATURED COMPANY
Click HERE to download a printable version of this newsletter.
Using Cells for Healing
RepliCel Life Sciences Inc. (OTCQB: REPCF) (TSXV:RP) is a regenerative medicine company developing cell therapies to treat conditions related to a deficit of healthy cells needed to heal and restore function.
Stem Cell Therapies With Major Catalysts Ahead
 Dr. George S. Mack
Dr. George S. Mack
Managing Director, BioDecade
The single most important share-price driver in the small- or micro-cap biotech space is the presence of a near-term catalyst that could boost share price. The smaller a company’s market valuation, the greater the potential impact of market-moving data. Many times anticipated good news is already baked into the share price, but that is not the case with Vancouver, B.C.-based RepliCel Life Sciences Inc., which has a microcap valuation of just $US19.8 million.
RepliCel has a stem cell franchise, currently in phase 1 and phase 2 development, that will put hair on your shiny bald head, heal your debilitating and painful Achilles heel and smooth the tired-looking wrinkles off your face. If any one of these technology platforms gets regulatory approval and then makes it to the market, that lowly market cap could grow into many multiples of what it is today. News, in fact, is on the way, including data from two clinical trials in the first quarter of this year. The company also has a hand-held dermal injector device that could be marketed next year.
Here’s the story.
From the normal hair follicle on a patient’s neck, RepliCel is advancing two cell technologies that are being developed in three different indications.
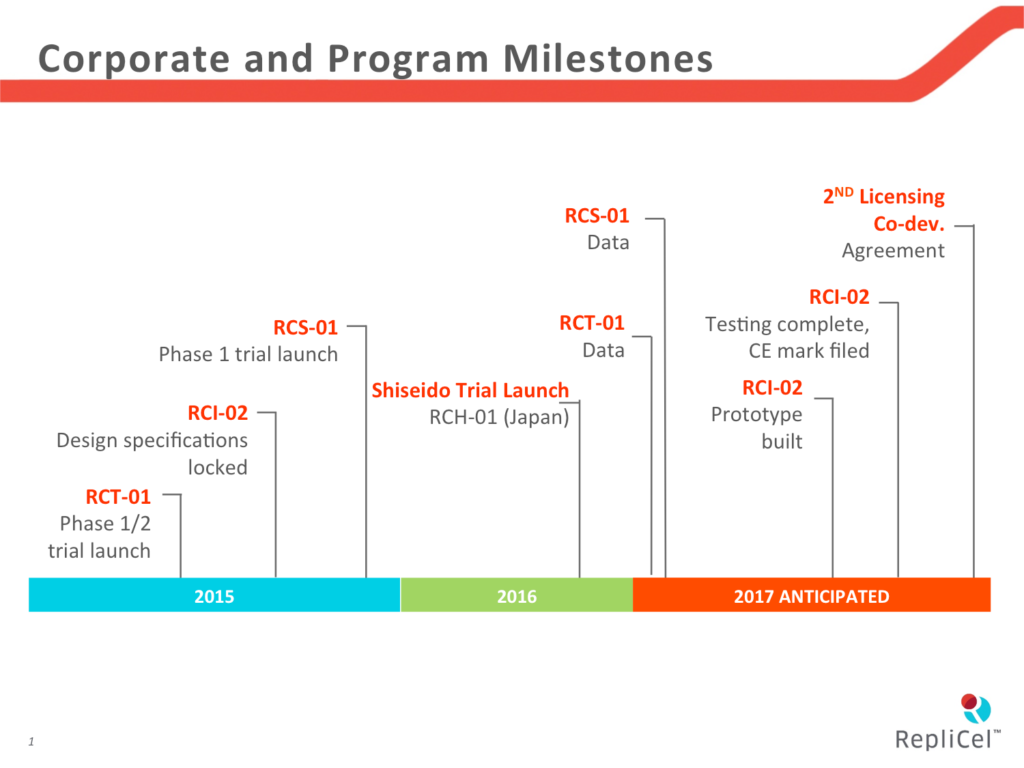
 Fibroblasts are harvested from the non-bulbar dermal sheath (NBDS) located on the long axis of the hair follicle (see figure at left). These cells are plentiful and are active producers of type 1 collagen, a protein made of 19 amino acids and which make up connective tissue, such as tendon, muscle and scar tissue. After preparation into doses, NBDS cells slated for use in chronic tendinosis are noted as RCT-01 for the Achilles heel indication. For the skin aging, sun damage and wrinkle indication, they are noted as RCS-01. These cells are harvested, isolated and mixed with a growth medium and expanded ex vivo, or outside the patient, for up to eight weeks in a batch especially for the patient from whom they were taken. This is the “autologous” model, where the patient is his or her own donor, which eliminates the possibility of tissue rejection. The tendinosis indication is in phase 1/2 and the skin study is in phase 1.
Fibroblasts are harvested from the non-bulbar dermal sheath (NBDS) located on the long axis of the hair follicle (see figure at left). These cells are plentiful and are active producers of type 1 collagen, a protein made of 19 amino acids and which make up connective tissue, such as tendon, muscle and scar tissue. After preparation into doses, NBDS cells slated for use in chronic tendinosis are noted as RCT-01 for the Achilles heel indication. For the skin aging, sun damage and wrinkle indication, they are noted as RCS-01. These cells are harvested, isolated and mixed with a growth medium and expanded ex vivo, or outside the patient, for up to eight weeks in a batch especially for the patient from whom they were taken. This is the “autologous” model, where the patient is his or her own donor, which eliminates the possibility of tissue rejection. The tendinosis indication is in phase 1/2 and the skin study is in phase 1.
Dermal sheath cup cells (DSCs) are located at the lower, rounded, fat or bulbar region of the follicle, and they support colonies of dermal papillae (DP) cells. Arterioles and capillaries in this region nourish and support these cells with oxygen and nutrients such as glucose, and hair fibers are generated and grow out from this dermal papilla area. The cells that will ultimately become the RCH-01 cell preparation are isolated and harvested from this region, and they are expanded ex vivo, in much the same way as RCT-01 and RCS-01 cells. The RSH-01 preparation is being developed for androgenic alopecia, better known as pattern baldness, and this project is in phase 2 in the U.S. It is also in clinical trials in Japan with global partner Shiseido.
The testing phase is completed for the dermal injector, designated as RCI-02, and an application has been filed in the European Union for the CE mark, which is displayed on medical devices that meet the requirements and standards that the appropriate regulator has set. The CE mark means approval and is similar the FDA’s 510(k).
Here are the catalysts or milestones RepliCel investors should be anticipating:
- Results from the company’s chronic tendinosis trial with RCT-01 as well as its skin aging, wrinkling and sun-damaged skin study with RCS-01 are imminent. The data are being crunched now, and the company’s update of January 5, 2017 indicates that these data will be published mid-way through the first quarter of this year. That implies that we should hear something in the mid-February range. The company says the data are being analyzed now by an independent third party.
- The company will complete fabrication of its dermal injector functional prototypes during 2017, and this is expected to be sufficient for the CE mark application to get the device approved in the European Union. RepliCel is going for a label approval for injection of hyaluronic acid-based dermal fillers, which are used by dermatologists and plastic surgeons to fill and smooth wrinkles and age lines of the face.
Market opportunity
An analyst could show investors the size of these markets—skin aging, Achilles heel and hair restoration—and any of the three could represent total markets worth billions of dollars. There is an opportunity to carve out hundreds of millions in revenue to produce an extraordinary, eye-opening top line. I prefer to focus on RepliCel’s current minimalist market cap of under $US20 million and the fact that movement of these clinical studies into phase 2 and phase 3 could mean a very significant increase in share price. RepliCel represents an undiscovered opportunity, but its low market cap precludes mutual funds and pensions from owning it. So, small hedge funds, family offices and individual investors should give this company a serious look.
Analyst Commentary
“RepliCel Life Sciences recapitalization provides funds to drive non-bulbar dermal sheath cell platform forward with renewed vigor Ð Spec BUY… RepliCel made a substantial advance on reducing its financial risk yesterday by closing on a $4.3M unit offering that will add about $4.1M in net cash to its balance sheet (current cash balance was probably at/near nil, so adjusted cash is probably at that level at month-end) and 8.2M shares/8.2M warrants to capital structure, bringing basic S/O in our model to 14.9M and fully diluted S/O to 26.2M.”
—Douglas W. Loe, PhD MDA, Echelon Wealth Partners,
November 1, 2016
Learn more about Echelon Wealth Partners
Disclosure and Declaration
Dr. George S. Mack, the author of this report, has no long or short position in any stock mentioned in this report. Other than information available on its website, RepliCel has had no input into the preparation of this report.
About the Author
Dr. George S. Mack is an analyst at consulting firm BioDecade where he is managing director. He performs biotechnology equity research for investors. His focus areas are oligonucleotide, cell and gene therapies. He has been a contributor to financial publications that include Buyside Magazine and Wall Street Research, where he wrote for institutional investors. Dr. Mack is a 1970 graduate of the University of South Carolina in Columbia where he earned his BS degree in biology and a 1974 graduate of the Medical University of South Carolina in Charleston where he earned his DMD degree. He can be reached at gmack@biodecade.com.
Disclosures
Investors and others should note that RepliCel Life Sciences posts important financial information using the investor relations section of the RepliCel Life Sciences website, replicel.com, and Securities and Exchange Commission filings.
The information contained in this facsimile message is intended only for the use of the individuals to whom it is addressed and may contain information that is privileged and confidential. If the reader of this message is not the intended recipient, you are hereby notified that any dissemination, distribution or copying of this communication is strictly prohibited. If you have received this communication in error, please notify us immediately by telephone at (707) 933-8500.
The material, information and facts discussed in this report are from sources believed to be reliable, but are in no way guaranteed to be complete or accurate. This report should not be used as a complete analysis of the company, industry or security discussed in the report. This is not an offer or solicitation of the securities discussed. Advisor-Access LLC and/or its employees, contractors and owners, may purchase or sell the securities mentioned in this report from time to time. Any opinions or estimates in this report are subject to change without notice. This report contains forward-looking statements that can be identified by the use of words such as “expect,” “intend,” “potential.” Forward-looking statements are predictions based on current expectations and assumptions regarding future events and are not guarantees or assurances of any outcomes, results, performance or achievements. You are cautioned not to place undue reliance upon these statements. These forward-looking statements are subject to a number of estimates and assumptions, and known and unknown risks, uncertainties and other factors. RepliCel Life Science’ actual results may vary materially from those discussed in the forward-looking statements as a result of factors and uncertainties disclosed in RepliCel Life Science’ reports filed with the Securities and Exchange Commission, which should be reviewed together with these forward-looking statements. The securities discussed may involve a high degree of risk and may not be suitable for all investors. Dr. George S. Mack had final approval of the content and is wholly responsible for the validity of the statements and opinions.
Please add “donotreply@advisor-access.com” to your address book and whitelist us.
CLICK HERE if it’s okay to share your name with this company.
CLICK HERE if you don’t want your name shared with the company.
CLICK HERE to confirm your subscription to Advisor Access.
About Advisor Access
Advisor-Access LLC was designed to bring compelling investment ideas to investors in the form of in-depth interviews with company management and the latest fact sheets and corporate presentations, in a concise format: the critical pieces of information an investor needs to make an informed investment decision. Read the Advisor-Access Full Disclosure Online